Evaluation of Sequencing of Anthracyclines and Taxanes for Locally Advanced HER2-negative Breast Cancer
Type of Study: Clinical Trial
Sponsor / Support: LACOG / GBECAM
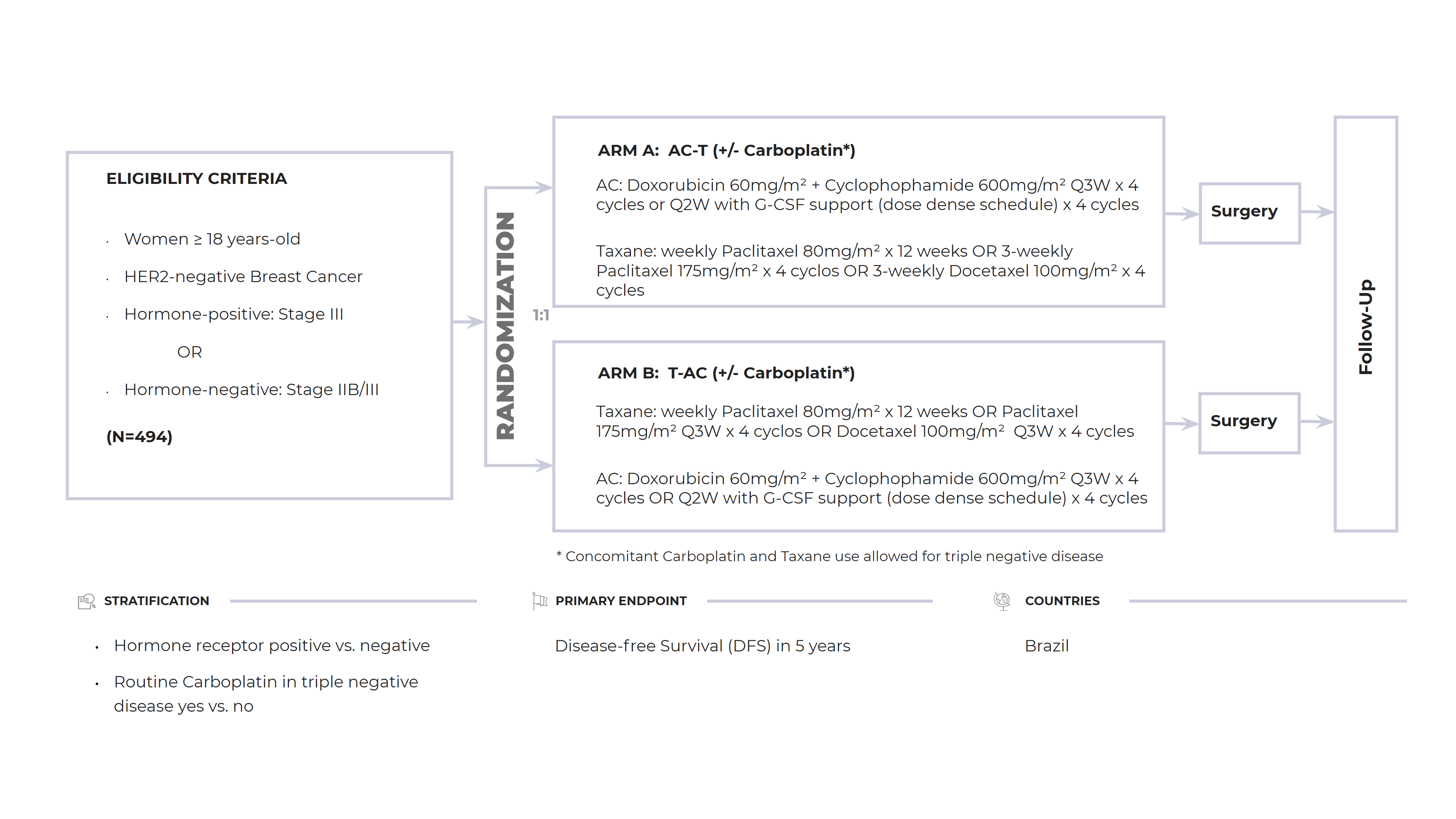
Primary Objectives: To evaluate in a phase III randomized clinical trial the comparison between an anthracycline-initiated neoadjuvant chemotherapy regimen (AC-T) versus a taxane-initiated regimen (T-AC) in patients with locally advanced HER2- breast cancer
Design: Phase III, open-label, multicenter randomized clinical trial
Sample Size: 494 patients
Principal Investigator: José Bines
Countries LATAM: Brazil
Clinicaltrials.gov Identifier: NCT04540692
The study is open to patients participation in the following research sites:
INCA – Instituto Nacional de Câncer / Rio de Janeiro / RJ / Brazil
Hospital de Clínicas de Porto Alegre / Porto Alegre / RS / Brazil
CEPON – Centro de Pesquisas Oncológicas / Florianópolis / SC / Brazil
Hospital do Câncer de Barretos / Barretos / SP / Brazil
ICTR – Instituto do Câncer e Transplante de Curitiba / Curitiba / PR / Brazil
Unesp – Universidade Estadual Paulista / São Paulo / SP / Brazil
CEON – Centro de Oncologia / Curitiba / PR / Brazil
UNICAMP – Universidade Estadual de Campinas / Campinas / SP / Brazil
São Camilo Oncologia / São Paulo / SP / Brazil
Hospital Araújo Jorge / Goiânia / GO / Brazil
Hospital Jardim Amália / Volta Redonda / RJ / Brazil
IMIP – Instituto de Medicina Integral Professor Fernando Figueira / Recife / PE / Brazil
CPCO – Centro de Pesquisa Clínica em Oncologia / Cachoeiro de Itapemirim / ES / Brazil